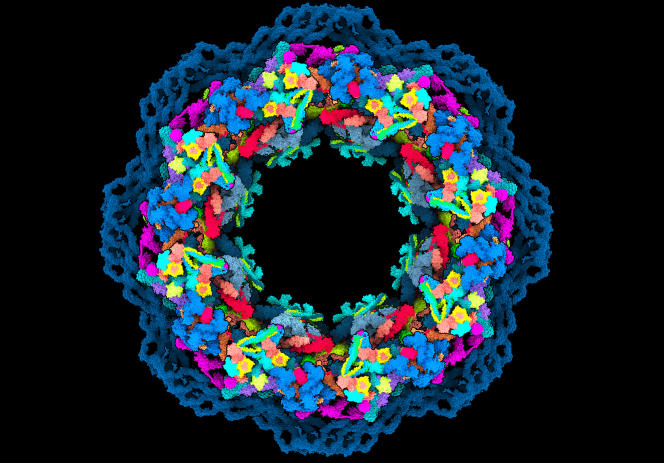
The nuclear pore, this ring-shaped structure which has the function of filtering the molecular exchanges between the nucleus of our cells and the cytoplasm in which it bathes, is of an appalling complexity. It is made up of about a thousand proteins divided into about thirty varieties of nucleoporins, intimately linked to each other to selectively close or open the passage. On June 9, the journal Science published a series of articles describing with unprecedented precision the intimate mechanisms of this filter. One of the studies combined the techniques of crystallography and electron cryomicroscopy with artificial intelligence (AI) to reconstruct 90% of this puzzle in 3D. AlphaFold-2, an AI program specializing in protein folding prediction, developed by the company DeepMind, was “a breakthrough for us”, says Agnieszka Obarska-Kosinska, a postdoctoral fellow at EMBL Hamburg, who carried out the modelization.
The nuclear pore, an almost reconstructed molecular puzzle